Fda Gmp Food Manufacturing Floor Requirements

Current food good manufacturing practices gmps are published in title 21 of the code of federal regulations part 110 21 cfr 110.
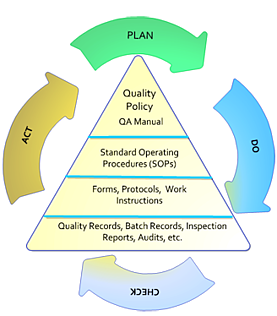
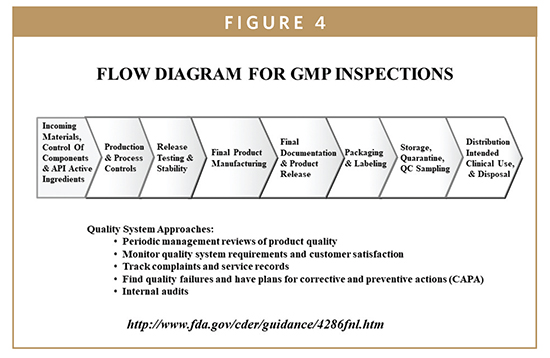
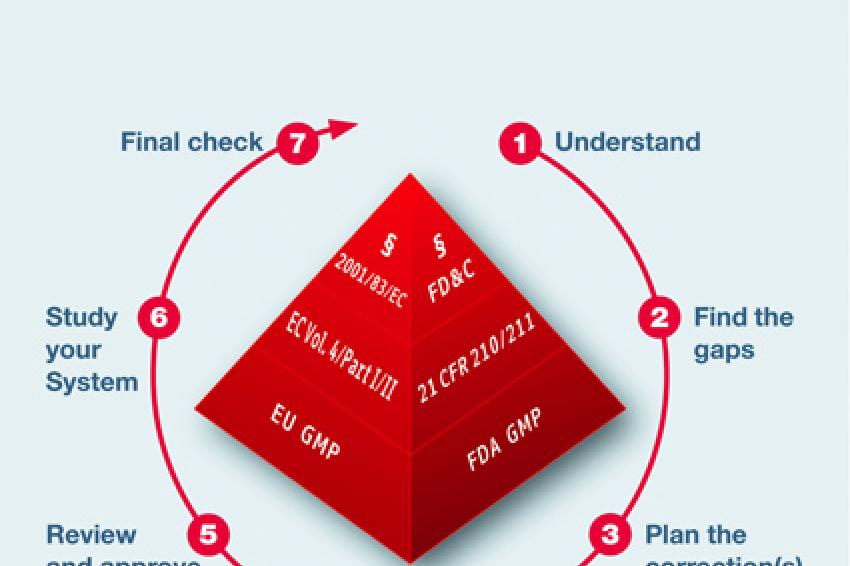
Fda gmp food manufacturing floor requirements. Gmps describe the methods equipment facilities and controls. The cgmp regulations for drugs contain minimum requirements for the methods facilities and controls used in manufacturing processing and packing of a drug product. Cgmp regulations generally address matters including appropriate personal hygienic practices design and. Following current good manufacturing practices cgmps help to ensure the safety of food.
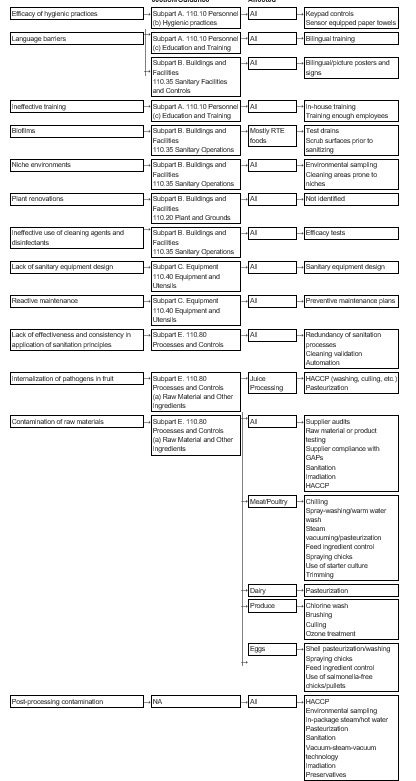
Subpart b buildings and facilities 110 20 plant and grounds. Subpart a general provisions 110 3 definitions. B defect action levels are established for foods whenever it is necessary and feasible to do so. 110 37 sanitary facilities and controls.
110 10 personnel. These guidelines provide minimum requirements that a manufacturer must meet to assure that their. 110 19 exclusions. 110 35 sanitary operations.